Abstract
Introduction : High-dose therapy (HDT) combined with autologous stem cell transplantation (ASCT) is a standard front-line treatment strategy for patients with newly diagnosed multiple myeloma (MM). Unfortunately, almost all the patients will eventually relapse, emphasizing the need for new treatment options or strategies to improve outcomes in these patients. A recent meta-analysis clearly demonstrated that post-transplant lenalidomide maintenance improves overall survival (OS), conflicting results were reported on the benefit of early post-transplant consolidation with bortezomib, lenalidomide, and dexamethasone (VRD). The aim of this study was to assess the effect of VRD consolidation on complete remission (CR) rate at Day100 post ASCT, a surrogate marker for progression free survival (PFS) and OS.
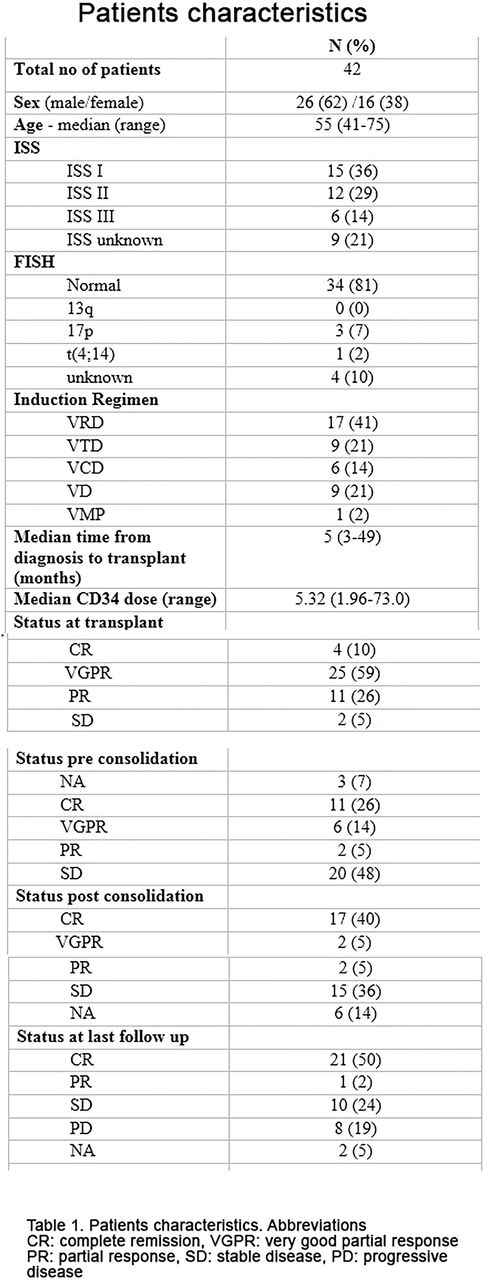
Methods: This study was conducted at the American university of Beirut Medical Center, Lebanon. A total of 42 patients newly diagnosed with MM had received ASCT, followed by a total post-transplant treatment consisted of 2 cycles of VRD consolidation and maintenance lenalidomide. These patients were treated and followed between 2011 and 2016. All patients received pre-transplant bortezomib based induction. Of them, 17 (41%) patients had VRD triplet combination. The conditioning regimen consisted of high dose melphalan. The consolidation regimen consisted of bortezomib 1.3 mg/m2 I.V. (days 1, 8, 15 and 22 of each 28-day cycle), lenalidomide 25 mg orally daily for 21 days every 28 days, dexamethasone 20 mg total orally (days 1, 8, 15 and 22 of each 28-day cycle) for a total of 2 cycles. Patients and disease characteristics are summarized in table 1.
Results: Median age at transplantation was 55 (41-75) years. Median time from diagnosis to transplant was 5 (3-49) months. The median number of days to reach an absolute neutrophil count more than 500/mm3 post-ASCT was 11 days. VRD consolidation therapy was initiated at a median of 59 (34-201) days from transplantation. In total, 40 (95%) patients completed 2 cycles of VRD without grade 3/4 adverse events. One patient could not receive bortezomib during cycle 2 because of severe peripheral neuropathy. All patients received maintenance with lenalidomide. At time of transplant, only 4 (10%) patients were in complete remission (CR), 25 (59%) patients in very good partial remission (VGPR), and 11 (26%) patients in partial remission. Day 30 evaluation post-transplant, 11 (26%) patients were in CR, 6 (14%) patients in VGPR. Importantly, assessment of disease status at day 100 post-transplant identified 17 (40%) patients in CR. After a median follow up of 26 months, the 2 years PFS was 81% and OS was 92.9%.
Conclusion: VRD consolidation significantly upgrades the CR rate after ASCT in patients with MM. These encouraging findings need to be verified and confirmed in prospective randomized trials, establishing a new standard of post-transplant treatment in MM.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal